Abstract
Dysregulated inflammatory responses are characterized by inappropriate levels of inflammatory markers, speed of generation, degree, and major site of production, such as a vital organ. COVID-19 severity and mortality are strongly associated with interleukin (IL)-6 levels. High IL-6 levels are also observed in idiopathic Multicentric Castleman Disease (iMCD). Previously, we developed the first anti-IL-6 monoclonal antibody (mAb) treatments and showed that C-reactive protein (CRP) production could be fully controlled by IL-6 in humans. Using mathematical modeling, with CRP as an IL-6 surrogate marker, we predicted the ability of an anti-IL-6 mAb to block plasma IL-6 activity and showed IL-6 inhibition was dependent on the extent of whole-body IL-6 production. We postulate that in patients (pts) in whom IL-6 concentration at the site of inflammation is higher than in the plasma, full blockade of plasma IL-6 activity, shown by complete CRP inhibition, is the minimum requirement to achieve clinical efficacy.
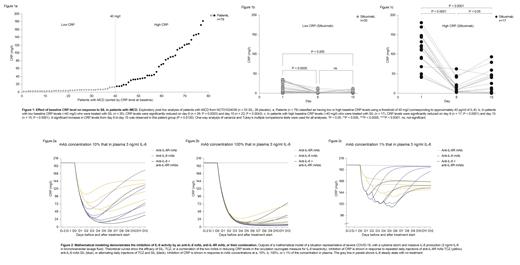
CRP inhibition with tocilizumab (TCZ) in pts with COVID-19. We identified 35 published studies evaluating the efficacy or potential role of anti-IL-6 therapy in pts with severe COVID-19. Surprisingly, only one (Luo et al. J Med Virol 2020;92:814) reported dynamics of CRP reduction for individual patients throughout treatment. We fitted a hypothetical curve of CRP reduction required to completely block IL-6, based on the half-life of CRP, and added data points of CRP serum levels from pts treated with anti-IL-6 receptor (IL-6R) therapy, TCZ, in this study. Complete reduction of CRP was not achieved in all patients: 3 pts who died had CRP levels ≥12.8 mg/l. Of 2 pts whose disease worsened, CRP levels were 93.5 mg/l and 6.3 mg/l. However, pts whose condition stabilized (n=9), or whose symptoms improved (n=1), had CRP levels close to the theoretical curve that is likely required to fully block IL-6 (CRP levels ≤5.0 mg/l). Half of these pts had been given repeated doses of TCZ.
Siltuximab (SIL) treatment (NCT01024036 trial) for pts with iMCD. To assess the impact of baseline CRP levels on response to anti-IL-6 therapy, SIL, patients were divided into low and high CRP groups based on a baseline CRP level threshold of 40 mg/l (corresponding to ~40 pg/ml of IL-6; Fig 1). In patients with low baseline CRP levels (<40 mg/l; n=35), CRP levels were significantly reduced with SIL treatment on day 8 (n=26; P=0.0003) and day 15 (n=22; P=0.0043) post-dosing. In patients with high baseline CRP levels (>40 mg/l; n=17), CRP levels were significantly reduced on day 8 (n=17; P<0.0001) and day 15 (n=15; P<0.0001) post-dosing (Fig 1). A significant increase in CRP levels from day 8 to day 15 was observed in these patients (P=0.0120); this increase was not observed in patients with low baseline CRP levels (P=0.243; Fig 1). There was a negative correlation between maximum CRP and hemoglobin change (P<0.001).
Inhibition of IL-6 activity by anti-IL-6 (SIL 700 mg) and anti-IL-6R (TCZ 800 mg) as monotherapy, intensified, or combined therapy. To evaluate the effect of therapy on IL-6 activity, our algorithm modeled inhibition of IL-6-(IL-6R/soluble IL-6R)-gp130 transducer complexes. This serves as a proxy for IL-6 bioactivity and accounts for the buffering capacity of gp130, which can be overwhelmed in inflammatory situations with high IL-6 concentrations. Due to uncertainty over the concentration of mAbs in alveoli relative to plasma, we modeled the effects of SIL and TCZ at local concentrations of 100%, 10%, and 1% (Fig 2) of those in plasma. Our model demonstrated that anti-IL-6 was associated with stronger inhibition of CRP than anti-IL-6R. However, only the association of both anti-IL-6 and anti-IL-6R mAbs was associated with a total blockade of CRP, which is probably necessary when IL-6 levels are associated with high risk to the patient. Different administration schedules to intensify anti-IL-6 therapy were modeled, including the repeated or combined use of anti-IL-6 and anti-IL-6R mAbs. The results form a basis to optimize treatment strategies to avoid the cytokine storm in several diseases, including cancer, iMCD, and autoimmune disorders. The feasibility of the theoretically defined approaches needs to be evaluated, particularly the potential side-effect profile for a combined treatment approach.
In conclusion, in clinical practice, IL-6 inhibition should be individualized based on pathophysiology and regular CRP monitoring.
Rossi: E-SANA Inc: Other: Co-founder of E-SANA Inc ; EUSA Pharma: Consultancy; LEO Pharma: Consultancy; NPO Petrovax Pharm: Consultancy. Levon: E-SANA Inc: Other: Co-founder of E-SANA Inc. Kanhai: EUSA Pharma: Current Employment. Fajgenbaum: EUSA Pharma: Research Funding; N/A: Other: Holds pending provisional patents for 'Methods of treating idiopathic multicentric Castleman disease with JAK1/2 inhibition' and 'Discovery and validation of a novel subgroup and therapeutic target in idiopathic multicentric Castleman disease'; Pfizer: Other: Study drug for clinical trial of sirolimus.
Siltuximab is approved for the treatment of iMCD. Tocilizumab is approved for the treatment of Rheumatoid arthritis, Giant cell arteritis, Cytokine release syndrome, Systemic juvenile idiopathic arthritis and Polyarticular juvenile idiopathic arthritis. Combination therapy using Siltuximab and Tocilizumab has not yet been approved.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal